(04/12/2014) | NikiasNews.gr | NIKIAS
Abstract
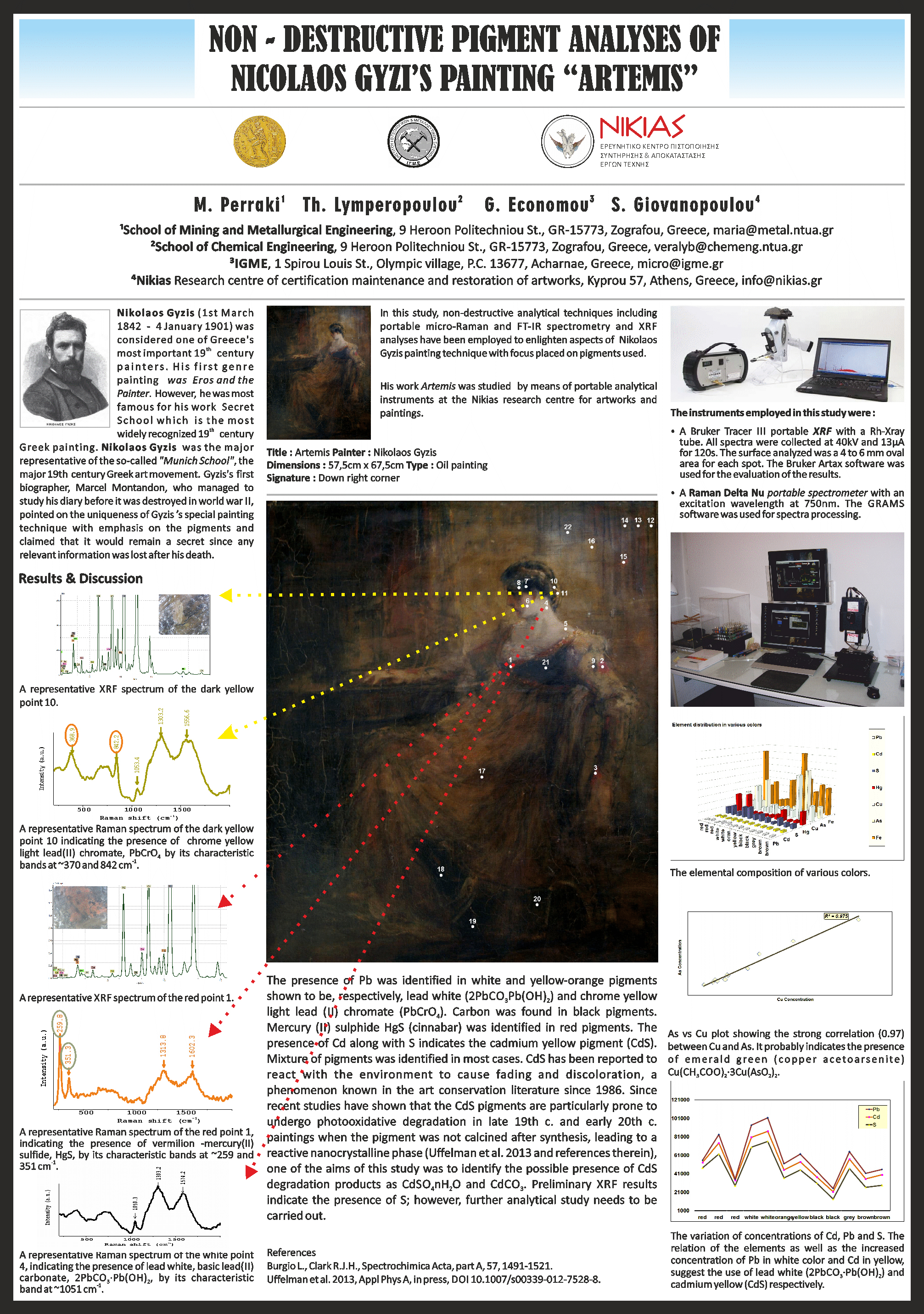
Non-destructive analytical techniques including portable micro-Raman and FT-IR spectrometry and XRF analyses have been employed to enlighten aspects of Nikolaos Gyzis (1 March 1842 - 4 January 1901) painting technique with focus placed on pigments used. His work Artemis was studied using a Raman Delta Nu spectrometer (excitation wavelength at 750 nm), a Perkin-Elmer Spectrum Two FT-IR spectrometer and a Bruker Tracer I1I-SD portable XRF (40kV) at the Nikias research centre for artworks and paintings.
The presence of Pb was identified in white and yellow-orange pigments shown to be. respectively, lead white (2PbC03*Pb(OH)2) and chrome yellow light lead (II) chromate (PbCr04). Carbon was found in black pigments. Mercury (II) sulphide HgS (cinnabar) was identified in red pigments. The presence of Cd along with S indicates the cadmium yellow pigment (CdS). Mixture of pigments was identified in most cases. CdS has been reported to react with the environment to cause fading and discoloration, a phenomenon known in the art conservation literature since 1986. Since recent studies have shown that the CdS pigments are particularly prone to undergo photooxidative degradation in late 19th c. and early 20th c. paintings when the pigment was not calcined after synthesis, leading to a reactive nanocrystalline phase (Uffelman et al. 2013 and references therein), one of the aims of this study was to identify the possible presence of CdS degradation products as CdSO4* nH2O and CdCO3. Preliminary XRF results indicate the presence of S and O; however, this has yet to be documented by Raman and FT-IR analyses.
Uffelman et al. 2013, Appl Phys A, in press, DOI 10.1007/s00339-012-7528-8











